No Time to Waste
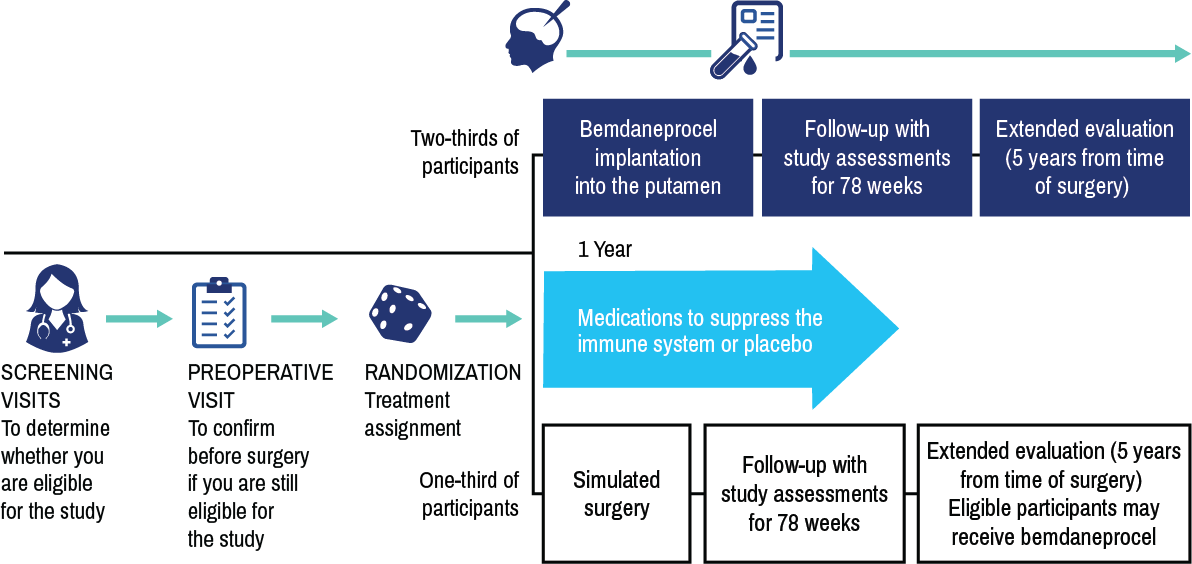
exPDite-2 is a double-blind, simulated surgery-controlled phase 3 study evaluating the potential efficacy and safety of an investigational cell therapy called bemdaneprocel (bem-da-nepro-cell).
Bemdaneprocel is currently being studied and is not approved for use in any country.
The sponsor of the exPDite-2 study is BlueRock Therapeutics.
About bemdaneprocel
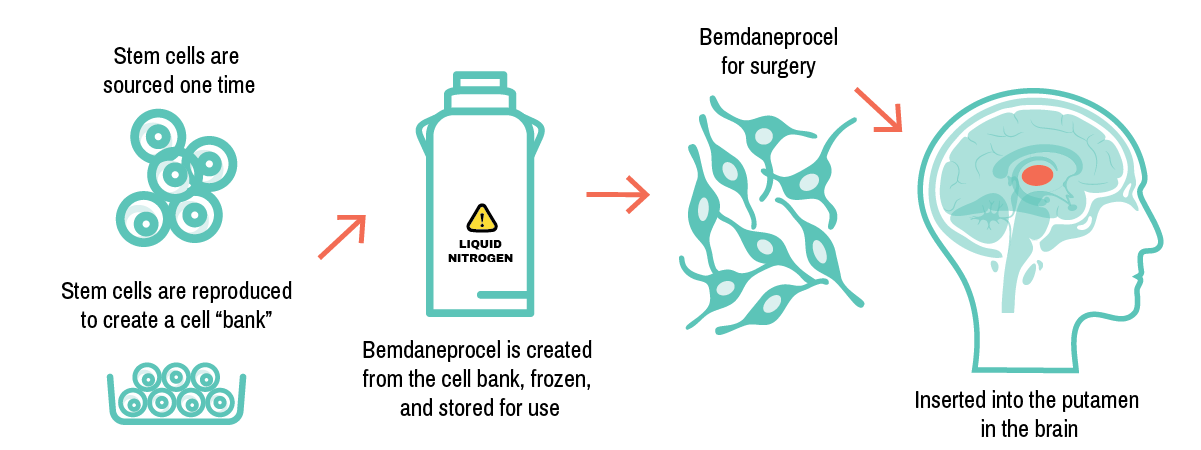
How is bemdaneprocel created?
BlueRock has developed a way to engineer dopaminergic neurons from stem cells. Bemdaneprocel contains the early form of these dopaminergic neurons, which are implanted in an area of the brain known as the putamen.
How is bemdaneprocel thought to work?
Bemdaneprocel is a cell therapy designed to replace the dopamine-producing neurons that are lost in Parkinson’s disease with the aim of engrafting (meaning integrate) into the brain to restore dopaminergic function.

Your brain contains neurons that make an important chemical called dopamine.
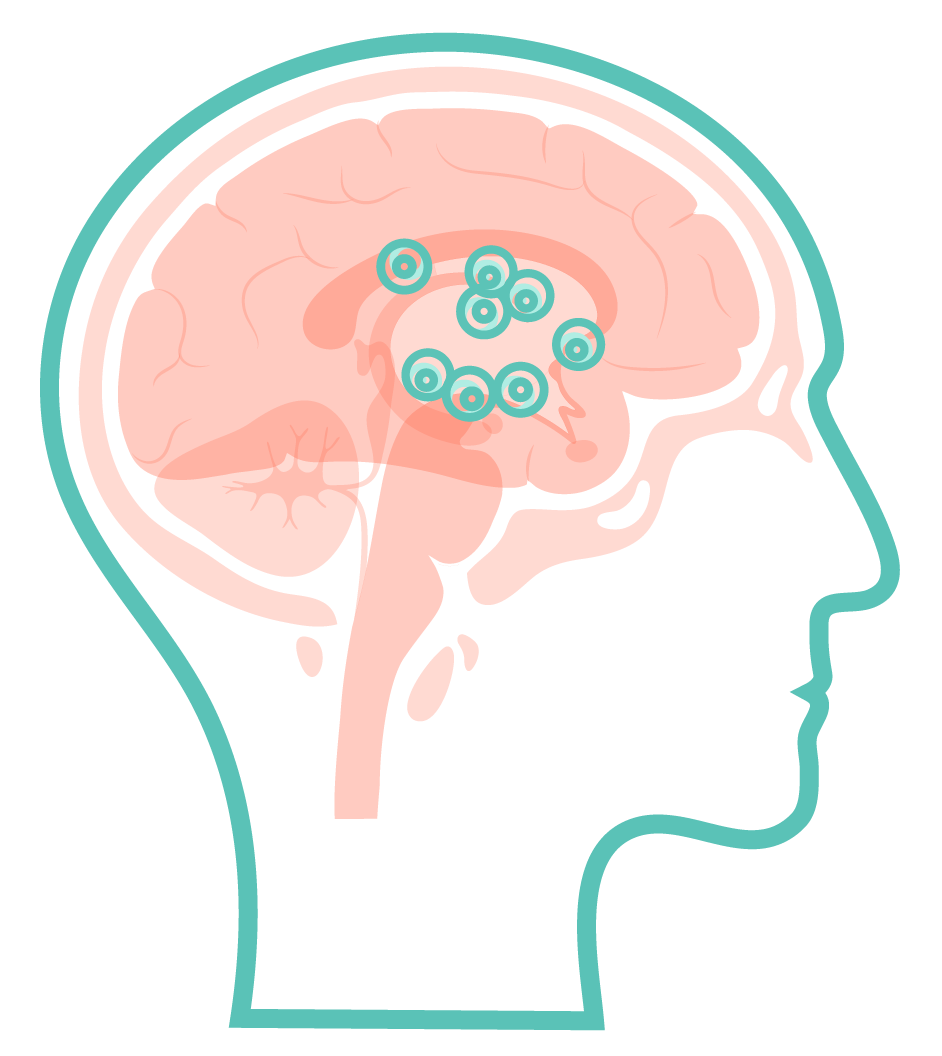
Parkinson’s disease causes these neurons to start to die, resulting in less dopamine production and causing the problems with movement and balance experienced by people living with Parkinson’s disease.
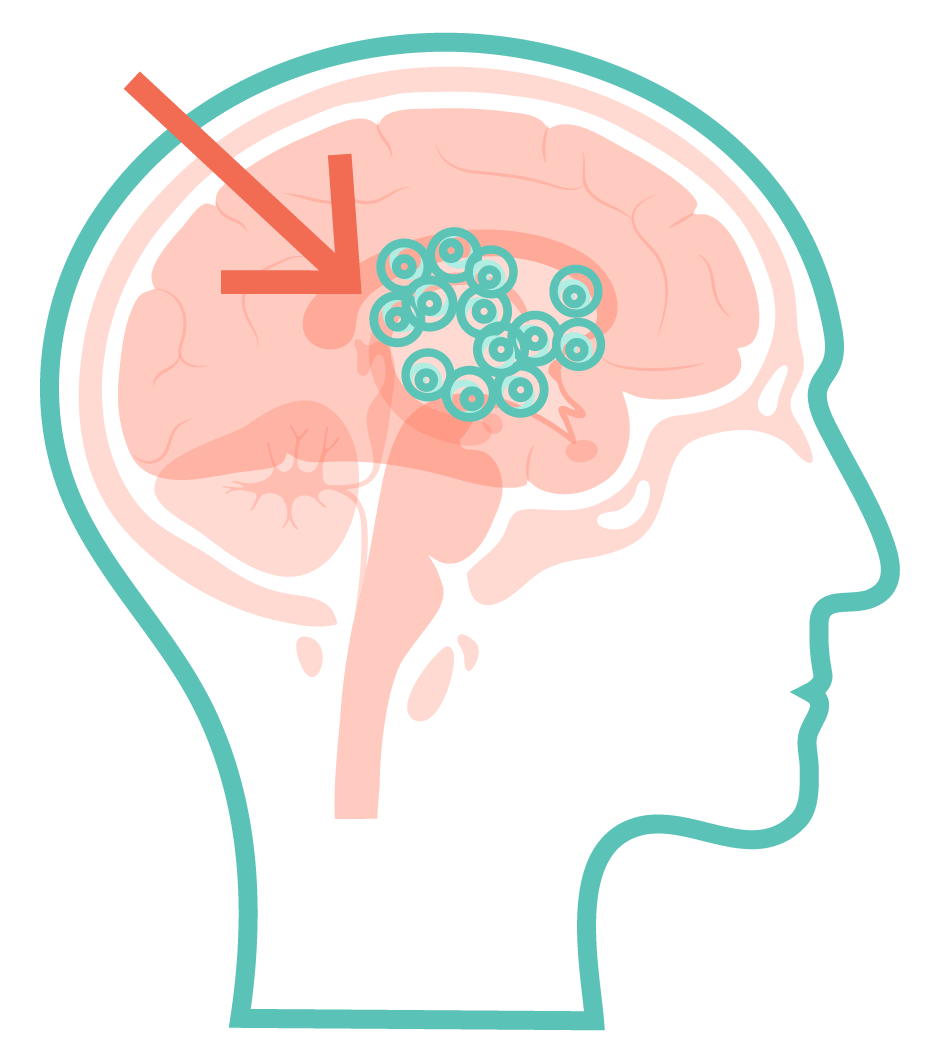
When implanted into your brain in a 1-time surgery, bemdaneprocel may replace these missing or damaged neurons with the aim of integrating into the brain to produce dopamine.
exPDite-2 will evaluate whether bemdaneprocel is effective in replacing lost dopaminergic neurons in people with Parkinson’s disease to improve motor symptoms and quality of life. In the phase 1 study, bemdaneprocel was well-tolerated.
Are you a good fit for exPDite-2?
Could I be considered for the exPDite-2 study?
You may be considered for the study
if you …
- were diagnosed with Parkinson’s disease 4–12 years ago.
- are 45–75 years old.
- are taking levodopa-based treatment to control motor symptoms.
- have at least 2.5 hours of OFF time each day.
You may not be able to take part if you ...
You may not be able to take part if you ...
- have had deep-brain stimulation (DBS).
- suffer from a condition such as multiple sclerosis, epilepsy, substance abuse, an infection, or had cancer in the last 5 years.
- are taking certain medicines that might affect the study results.
- have had gene or cell therapy, surgery or radiation therapy to your brain, or are unable to have a general anesthetic or surgery.
- are pregnant or breastfeeding.
A study doctor will tell you more about these requirements.
For qualified study participants, all treatments and tests may be provided at no charge. Support with travel associated with the study and reasonable reimbursement costs for you and your care partner may be provided throughout the exPDite-2 study. The study doctor and/or their staff will discuss this with you at your first visit.
How the study works
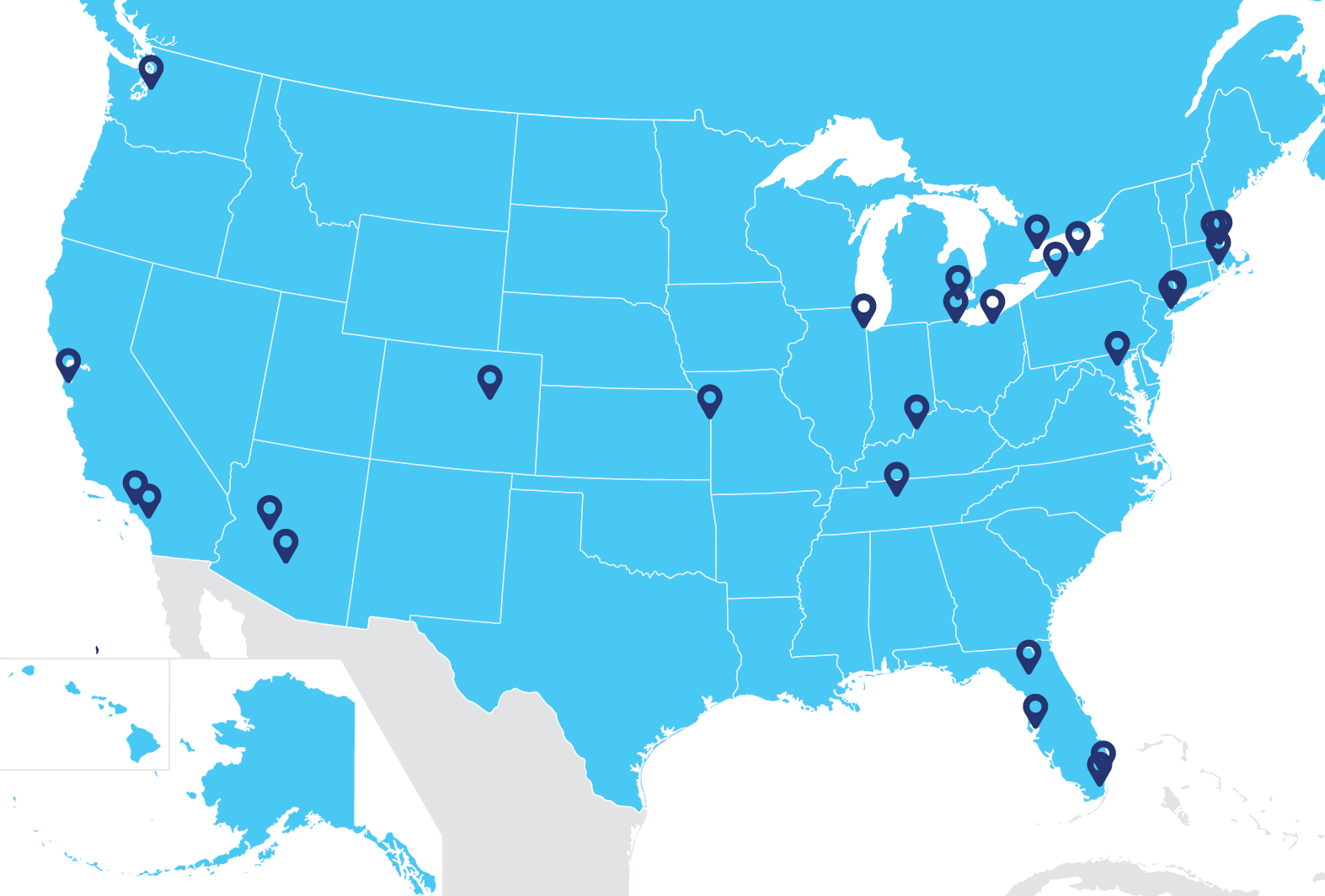
Study locations
Planned exPDite-2 study centers are shown on the map. You and your care partner may need to travel for MRIs and surgery. Reimbursement for travel associated with the study and reasonable expenses for you and your care partner may be available. All medication and procedures may be covered as part of the study.
All exPDite-2 study centers are shown on the map. You and your care partner may need to travel for MRIs and surgery. Reimbursement for travel associated with the study and reasonable expenses for you and your care partner may be available. All medication and procedures may be covered as part of the study.
No Time to Waste
Parkinson’s disease is a progressive neurodegenerative disorder involving a loss of dopamine-producing neurons in the brain.1
Currently available medications and therapies aim to ease symptoms and improve quality of life but do not address the underlying causes of the disease.2-4

Progression of Parkinson’s disease often requires the addition of levodopa-based treatments which can lead to increases in adverse reactions, including dyskinesia (or uncontrolled, involuntary movement) and other motor and nonmotor symptoms.5
Current treatments do not address the loss of dopaminergic neurons (an underlying cause of disease), do not affect disease progression, and lose effectiveness over time.1,6
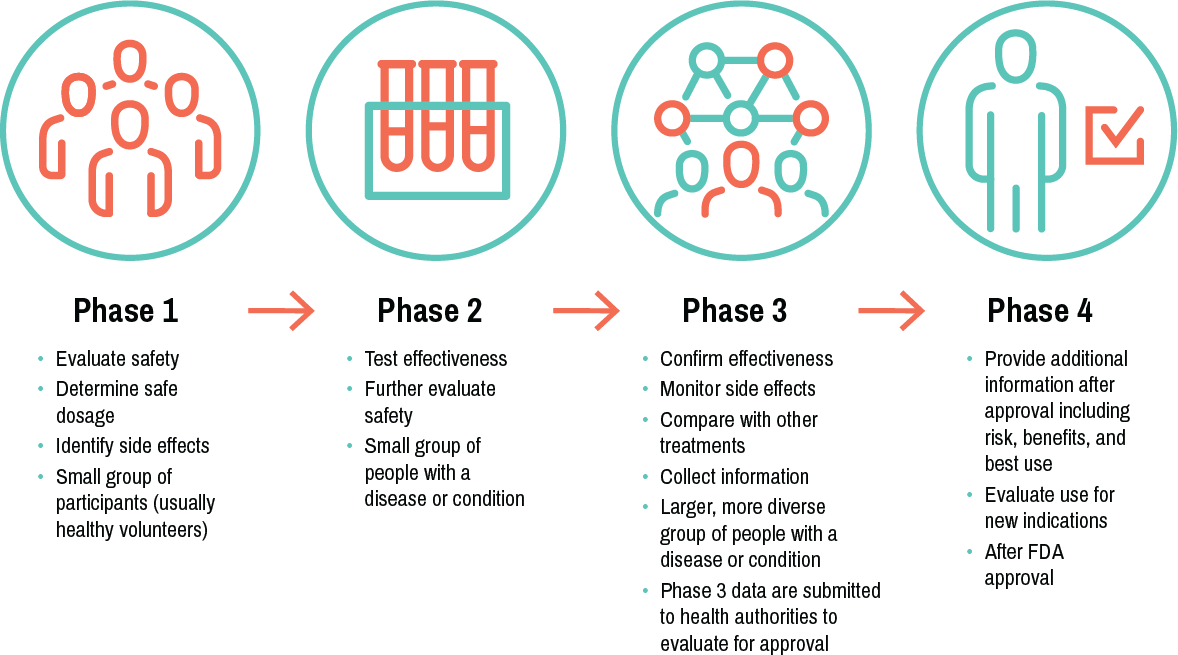
Clinical trial phases
here are generally 4 phases of clinical trials. Successful completion of each is required to advance to the next stage.
References: 1. Ramesh S, Arachchige A. AIMS Neuro. 2023;10(3):200-231. 2. Yiannopoulou KG, Papageorgiou SG. J. Cent Nerv Syst Dis. 2020;12:1179573520907397. 3. Livingston G, et al. Lancet. 2020;396:413–446. 4. NIH-NINDS. Parkinson’s Disease: Challenges, Progress, and Promise. https://www.ninds.nih.gov/current-research/focus-disorders/parkinsons-disease-research/parkinsons-disease-challenges-progress-and-promise. Accessed March 2024. 5. Pandey S, Srivanitchapoom P. Ann Ind Acad Neurol. 2017;20(3): 190-196. 6. Tambasco N, et al. Current Neuropharmacol. 2018;16:1239-1252.